Safety of Household, Commercial and Similar Electrical Appliances
Appliances for Skin Exposure to Optical Radiation
Strategic Recommendations for Appliances for Skin Exposure to Optical Radiation
- Start BIS Certification Process Now
Begin early to accommodate lab scheduling, documentation, and factory inspections before the enforcement deadline. - Partner with Compliance Experts
ERA’s regulatory specialists can help with application filing, lab coordination, and ISI marking compliance under Scheme-I. - Contact ERA Support Team
📧 cs@eraglobal.co.in | 📞 +91 9599296331 | 💬 WhatsApp
Reach out for QCO interpretation, BIS testing assistance, and Schedule A support. - Track Notifications & Circulars
Subscribe to the ERA Newsletter for real-time updates on BIS circulars, amendment notices, and industry alerts. - Ensure Marking Before Sale
Appliances must clearly display the ISI mark on each unit and its outer packaging prior to being marketed or sold.
| Section | Details |
|---|---|
| Product Name | Appliances for Skin Exposure to Optical Radiation |
| Applicable Indian Standard (IS) No. | IS 302 (Part 1):2024 |
| Title of Indian Standard | Household and Similar Electrical Appliances – Safety – Part 1: General Requirements |
| Quality Control Order | Safety of Household, Commercial and Similar Electrical Appliances (Quality Control) Order, 2025 |
| Notification & Amendments | Notified via S.O. 2232(E), dated 19 May 2025 Supersedes QCO 2024: S.O. 4098(E), dated 17 Sept 2024 |
| Final Enforcement Date | Large & Medium Enterprises: 19 March 2026 Small Enterprises: 19 June 2026 Micro Enterprises: 19 September 2026 |
| Objective & Scope | To regulate the safety of appliances emitting optical radiation for cosmetic or therapeutic skin treatments through BIS certification and conformity to Indian standards. |
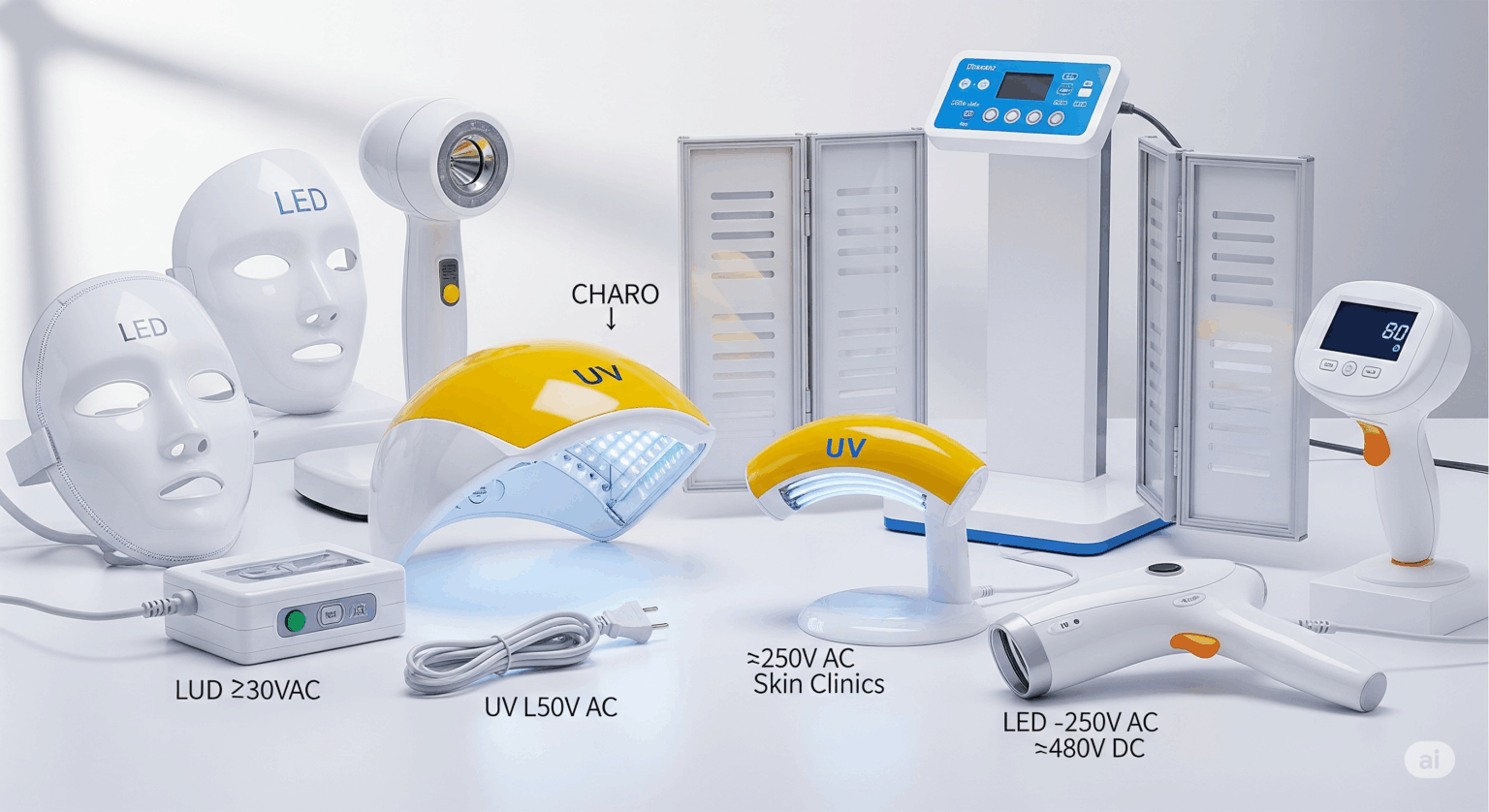
| Products Covered | Electrical appliances for skin treatment using UV, infrared, or visible light (e.g., tanning lamps, phototherapy devices), operated at ≤250V AC / ≤480V DC. |
| Exemptions | - Products exclusively for export - R&D imports (≤200 units/year) - Inventory under Schedule A allowed for sale within 6 months post enforcement |
| Industries Impacted | Beauty & Personal Care Device Manufacturers, Dermatology Equipment OEMs, Wellness Clinics, E-commerce Retailers |
| Mandatory Compliance | - Must comply with IS 302 (Part 1):2024 - BIS certification under Scheme-I is compulsory - ISI mark must be affixed on product and packaging |
| Next Steps for Stakeholders | Apply for BIS license, complete lab testing, affix ISI mark, declare non-compliant inventory under Schedule A |
| Legal Framework Provision & Enforcement | Enforced by BIS under the BIS Act, 2016 with authority to inspect, penalize, and seize non-compliant goods |
| Penalties | Non-compliance may result in seizure of stock, license suspension, fines, or imprisonment up to 2 years, or both |
| Conclusion | All appliances for optical radiation-based skin exposure must be BIS-certified under IS 302 (Part 1):2024 and ISI-marked from 19 March 2026. |
| References/Annexures | - QCO 2025: S.O. 2232(E) [19 May 2025] - Superseded QCO 2024: S.O. 4098(E) [17 Sept 2024] |
Ready to start your certification journey?
Let us help you navigate regulatory challenges and achieve certification with ease. Leave us your details, and we’ll get back to you—or request a free consultation today.
Get in touch with us today
Notification
Appliances for Skin Exposure to Optical Radiation are covered under the Safety of Household, Commercial and Similar Electrical Appliances (Quality Control) Order, 2025, notified by the Department of Consumer Affairs through S.O. 2232(E) dated 19 May 2025 under the BIS Act, 2016. This order replaces the earlier QCO notified via S.O. 4098(E) on 17 September 2024.
Overview
These appliances emit controlled doses of light (UV, IR, or visible spectrum) to provide cosmetic or therapeutic treatment for skin, commonly used in beauty salons, spas, or personal care routines. They pose potential risks if poorly manufactured, including burns, eye damage, and overexposure. Hence, strict safety standards are necessary.
Objective & Scope
The QCO mandates conformance to IS 302 (Part 1):2024, ensuring appliances meet essential safety parameters, including optical radiation limits, electrical insulation, moisture protection, and overheating safeguards.
Products Covered
- Home-use and professional skin therapy devices using UV/IR/LED light
- Tanning lamps, phototherapy units, cosmetic laser/light appliances
- Rated at ≤250V AC or ≤480V DC
Exemptions Provided
- Products manufactured exclusively for export
- Imports for research and development (≤200 units/year)
- Declared stock under Schedule A can be sold within 6 months of enforcement
Industries Impacted
- Personal care appliance brands
- Salon and spa device OEMs
- Dermatological therapy equipment suppliers
- Online platforms and retail chains in the beauty and wellness sector
Mandatory Compliance Requirements
- Certification under BIS Scheme-I is mandatory
- Appliances must fully comply with IS 302 (Part 1):2024
- Each product and package must display the ISI mark
Enforcement Timeline
- Large & Medium Enterprises: 19 March 2026
- Small Enterprises: 19 June 2026
- Micro Enterprises: 19 September 2026
Next Steps for Stakeholders
- Begin BIS license application and testing process
- Ensure sample testing at BIS-approved laboratories
- Declare unsold non-compliant stock under Schedule A
- Update labeling and packaging for ISI mark compliance
Legal Provisions, Enforcement & Penalties
This order is enforceable under the BIS Act, 2016 by the Bureau of Indian Standards.
Penalties for non-compliance include:
- Seizure of unmarked or uncertified products
- Suspension or cancellation of BIS license
- Fines and/or imprisonment up to 2 years, or both
Conclusion
Effective 19 March 2026, all appliances for skin exposure to optical radiation sold in India must be BIS-certified, conform to IS 302 (Part 1):2024, and bear the ISI mark. This move prioritizes user safety and ensures uniform quality across the market. Stakeholders should begin compliance processes immediately.
Ready to start your certification journey?
Let us help you navigate regulatory challenges and achieve certification with ease. Leave us your details, and we’ll get back to you—or request a free consultation today.
